Clinical Cancer Research | Research on Curcumin
Abstract
Curcumin, a polyphenolic antioxidant derived from a dietary spice, exhibits anticancer activity in rodents and in humans. Its efficacy appears to be related to induction of glutathione S-transferase enzymes, inhibition of prostag-landin E2 (PGE2) production, or suppression of oxidative DNA adduct (M1G) formation. We designed a dose-escala-tion study to explore the pharmacology of curcumin in humans. Fifteen patients with advanced colorectal cancer refractory to standard chemotherapies consumed capsules compatible with curcumin doses between 0.45 and 3.6 g daily for up to 4 months. Levels of curcumin and its metab-olites in plasma, urine, and feces were analyzed by high-pressure liquid chromatography and mass spectrometry. Three biomarkers of the potential activity of curcumin were translated from preclinical models and measured in patient blood leukocytes: glutathione S-transferase activity, levels of M1G, and PGE2 production induced ex vivo.
Dose-limiting toxicity was not observed. Curcumin and its glucuronide and sulfate metabolites were detected in plasma in the 10 nmol/L range and in urine. A daily dose of 3.6 g curcumin engendered 62% and 57% decreases in inducible PGE2 production in blood samples taken 1 hour after dose on days 1 and 29, respectively, of treatment compared with levels observed immediately predose (P < 0.05). A daily oral dose of 3.6 g of curcumin is advocated for Phase II evaluation in the prevention or treatment of cancers outside the gastroin-testinal tract. PGE2 production in blood and target tissue may indicate biological activity. Levels of curcumin and its metabolites in the urine can be used to assess general com-pliance.
The absence of sensitive markers of efficacy and compli-ance has frequently confounded the optimization of clinical trials of novel cancer chemopreventive agents, particularly in the case of mechanistically multitargeted diet-derived agents, such as flavonoids and other polyphenols. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; diferu-loylmethane], a major constituent of the yellow spice turmeric derived from the rhizomes of Curcuma spp., is one such poly-phenol. Curcumin has been shown to prevent cancer in the colon, skin, stomach, duodenum, soft palate, and breasts of rodents after oral administration (1–3). In clinical pilot studies in Taiwan and India, curcumin has been associated with regression of premalignant lesions of the bladder, soft palate, stomach, cervix, and skin, and with treatment responses in established malignancy (4, 5). Mechanisms by which curcumin prevents cancer are thought to involve up-regulation of carcinogen-detoxifying enzymes such as glutathione S-transferases (GST; refs. 6, 7), antioxidation (8, 9), and suppression of expression of the isoenzyme cyclooxygenase-2 (COX-2; refs. 10, 11).
The pharmacokinetic properties of curcumin in humans remain relatively unexplored. In rodents, curcumin undergoes avid metabolism by conjugation and reduction, and its disposi-tion after oral dosing is characterized by poor systemic bioavail-ability (9, 12). In a pilot study of a standardized oral Curcuma extract, doses up to 180 mg of curcumin per day were admin-istered to patients with advanced colorectal cancer for up to 4 months without overt toxicity or detectable systemic bioavail-ability (13). A subsequent study has suggested that doses up to 8 g could be administered daily to patients with premalignant lesions for 3 months without overt toxicity (4).
The aims of the study described here can be divided into three broad categories. Firstly, we sought to analyze in detail the toxicity of high doses of curcumin administered orally to pa-tients with advanced cancer. Expanding the reported data from the only published trial of doses 1.5 g daily in humans (4), we aimed to make this assessment by clinical parameters, quality-of-life questionnaire, and hematologic/biochemical tests. Sec-ondly, we aimed to investigate the systemic effects of curcumin consumption. On the basis of our data from preclinical studies in models of colorectal carcinogenesis and in blood from healthy volunteers, we selected three biomarkers for translation into early clinical trials of curcumin on account of their relevance to carcinogenesis and the magnitude of change in response to treatment (9 –11, 13). The three indices of the potential phar-macological activity of curcumin measured in patient blood leukocytes were: GST activity, levels of a deoxyguanosine adduct (M1G) formed via oxidative DNA damage, and inducible prostaglandin E2 (PGE2) levels as an indicator of COX-2 activ-ity induced ex vivo by lipopolysaccharide (LPS). GST enzyme activity has been shown to be up- or down-regulated in rat tissues after oral consumption of curcumin, depending on the dose and route of administration (6, 7, 9). M1G levels are related to lipid peroxidation and oxidative stress and can be altered by feeding rats curcumin in their diet or by dietary modification in human volunteers (9, 14). Curcumin down-regulates COX2 tran-scription in human-derived colon cells (10). When added in vitro to blood from healthy volunteers, curcumin (1 mol/L) reduced LPS-induced COX-2 protein levels and concomitant PGE2 production by 24% and 41%, respectively (11). The third aim of this Phase I study was to test the hypothesis that curcu-min or products of its metabolism can be detected in blood or excreta of humans using high-performance liquid chromatogra-phy (HPLC) with UV detection and tandem quadrupole mass spectrometry (MS; refs. 13, 15).
Overall, the study was designed to define pharmacokinetic and pharmacodynamic parameters, which might help to opti-mize the clinical evaluation of curcumin in Phase II chemopre-vention or chemotherapy trials.
Patients and Methods
Patients. The trial and formulation were approved by the local ethics committee and the United Kingdom Medicines Control Agency. Fifteen patients with histologically proven adenocarcinoma of the colon or rectum, for which no additional conventional therapies were available, met the following eligi-bility criteria: measurable or evaluable disease; age 18 years; WHO performance status of 0 to 2 and life expectancy 12 weeks; absolute neutrophil count $1.5 109/L; hemoglobin $10 g/dL; platelets $100 109/L; aspartate aminotransferase and alanine aminotransferase 2.5 times the upper limit of normal; serum bilirubin and creatinine 1.5 upper limit of normal; and no previous investigational or chemotherapeutic drugs within 28 days before enrolment. Exclusion criteria in-cluded: active chronic inflammatory or autoimmune disease; active infection, including viral infection; significant impair-ment of gastrointestinal function or absorption; active peptic ulcer disease; known biliary obstruction or biliary insufficiency, and use of nonsteroidal anti-inflammatory drugs within 14 days of enrolment. They were enrolled between December 2000 and December 2002 at the University Hospitals of Leicester. Pa-tients were asked to abstain from nonsteroidal anti-inflamma-tory drug use and the consumption of foods containing the spice turmeric during the study period, and their general practitioners were asked not to prescribe nonsteroidal anti-inflammatory drugs. Written informed consent was obtained from each patient before enrolment. Demographic and baseline characteristics of patients are shown in Table 1. All of the patients were white Caucasians except for 1 patient at the second dose level who was Indian.
Formulation, Dose, and Study Design. “C3” curcumi-noid capsules were provided in a single batch by the Sabinsa…
…Corporation (Piscataway, NJ). Each capsule contained 500 mg of curcuminoids (450 mg of curcumin, 40 mg of desmethoxy-curcumin, and 10 mg of bisdesmethoxycurcumin), confirmed by HPLC/MS. This formulation, which in the following text will be referred to as “curcumin,” was selected on account of its repro-ducibility of curcuminoid content and the curcumin dose, which allowed rapid dose escalation from the highest doses adminis-tered previously to patients with cancer. All of the capsules of a daily dose were consumed together with water in the morning after at least 2 hours of fasting. Dependent on dose level, patients consumed 1, 2, 4, or 8 capsules (containing 450, 900, 1800, or 3600 mg of curcumin) once daily, as shown in Table 1. The highest dose level was defined as the dose at which plasma levels of curcumin were detected or any pharmacodynamic effects were observed. Treatment was continued until disease progression was established or consent was withdrawn.
Clinical Measurements. Blood, urine, and feces were collected on days 1, 2, 8, and 29, protected from light and stored at 80°C. Blood collection was predose and at 0.5, 1, 2, 3, 6, and 8 hours after dose, and samples were kept in tubes pre-treated with lithium-heparin (Sarstedt, Loughborough, United Kingdom). Full blood cell count and urea, electrolytes, liver, and bone function were measured in venous samples, and phys-ical examination was performed, before treatment and on treat-ment days 1, 2, 8, 29, and monthly thereafter. Serum levels of total cholesterol and the tumor markers carcinoembryonic antigen, CA19.9, and CA125 were measured before treatment and every month of treatment. Blood samples for analysis of GST activity and M1G levels were collected 1 week before and on days 1, 2, 8, and 29 of treatment, immediately before dosing for M1G or immediately before and 1 hour after each dose for GST. Lymphocytes were separated from fresh blood using Ficoll-paque Plus (Amersham Pharmacia Biotech, Bucks, United Kingdom), resuspended in 1 mL of 10 mmol/L Tris-HCl (pH 7.8) and stored at 80°C. Patients completed the European Organization for Research and Treatment of Cancer quality of life questionnaire GLQ-C30 (version 2.0) pretreatment and monthly during treatment (16).
Patients were evaluated for tumor response every 8 weeks, using computed tomography or magnetic resonance imaging scanning, in addition to monthly chest X-rays. Measurements were made using the WHO Solid Tumor Response Criteria. All of the measurable, evaluable, and nonevaluable lesions were accounted for in the tumor assessment. Measurable lesions were quantified by the sum of the products of perpendicular diame-ters. Partial response was defined as at least a 50% decrease in the sum of the product of the perpendicular diameters of meas-urable lesions from baseline and with no development of new lesions. Progressive disease was defined as at least a 25% increase, clear worsening from previous assessment of any evaluable disease, reappearance of any lesion which had disap-peared, or appearance of any new lesion/site. Stable disease was defined as the scenario in which the disease status had neither responded to meet the partial response criterion nor progressed to meet the progressive disease criteria.
Pharmacodynamic Assessments. Glutathione and 1-chloro-2,4-dinitrobenzene were purchased from Sigma (Poole, United Kingdom). Once thawed, lymphocyte samples were son-icated for 30 seconds (Fisher 550 sonicator, Pittsburgh, PA) on ice and centrifuged at 3,000 g (15 minutes, 4°C). Total GST activity in the supernatant was measured spectrophotometrically using glutathione and 1-chloro-2,4-dinitrobenzene as substrates in triplicate for each sample (17). Results were corrected for protein levels using the Bio-Rad protein assay (Bio-Rad, Hemel Hempstead, United Kingdom). The GST activity values were quoted as nanomole 1-chloro-2,4-dinitrobenzene conjugated with glutathione per minute per milligram of lymphocytic pro-tein. The GSTM1, GSTT1, and GSTP1 genotypes were deter-mined by PCR methods described previously (18 –20). Murine M1G monoclonal antibody D10A1 was prepared as described previously (14). Antirabbit and antimurine horseradish peroxi-dase antibodies were purchased from Dako (Ely, United King-dom). M1G standards were synthesized and characterized, genomic DNA was extracted from whole blood, and leukocytic M1G adduct levels were analyzed by immunoslot blot in tripli-cate as described previously (9, 13, 14). Discrepancies in the amount of DNA per slot were corrected for by staining the nitrocellulose filter with propidium iodide and performing UV light densitometry (9, 13). The detection limit for M1G was 5 adducts per 108 nucleotides. Blood was also taken for assess-ment of plasma PGE2 concentration induced ex vivo as reported previously (11).
Measurement of Curcumin and Its Metabolites. Ex-traction of curcumin and its metabolites (curcumin glucuronide and curcumin sulfate) from plasma, urine, and feces; agent and metabolite recovery; and details of the reverse-phase HPLC (UV-visible detection) analysis were as described previously (13, 15). Retention times of curcumin glucuronide, curcumin sulfate, and curcumin were in general 24, 31, and 37 minutes respectively. The limit of detection for curcumin in plasma and urine was 5 pmol/mL. In most chromatographic analyses per-formed, curcumin and its conjugates were detected at levels that were close to the limit of quantitation. Quantitation was per-formed with a standard curve but without an internal standard and is, therefore, referred to as “semiquantitation.” Interday variation of the assay for curcumin was 7.0%. Results are presented as mean SD.
The identities of curcuminoids and their metabolites in urine and plasma were verified using an Agilent 1100 series HPLC with in-line Applied Biosystems/MDS SCIEX API 2000 ion spray tandem quadrupole MS. Separation of curcuminoids and their metabolites was achieved using an HPLC method published previously (13, 15). Identification of compounds was achieved by MS in negative ion mode. Compound-specific fragmentation was detected using multiple reaction monitoring to identify curcumin and curcumin glucuronide (m/z 367 to 134 transition), desmethoxycurcumin and desmethoxycurcumin glucuronide (m/z 336 to 119 transition), and curcumin sulfate (m/z 447 to 367 transition).
Statistical Evaluation. Results were subjected to ANOVA and linear regression analysis using Minitab (version 13) and SPSS (version 11.0) software packages. Plots of resid-uals were used to ensure that variances were homogeneous and that the residuals had a normal distribution. Comparison of samples taken immediately before dosing and 1 hour after dose was performed by paired t test for individual values, with Bonferroni adjustment for pair-wise comparisons between group means, and by repeated measures ANOVA for pooled measurements. Degrees of freedom (df) are stated for all of the results with < 0.05.
Results
Tolerability of Oral Curcumin. Curcumin was well tol-erated at all of the dose levels, and dose-limiting toxicity was not observed. Two types of gastrointestinal adverse events were reported by patients, which were probably related to curcumin consumption. One patient consuming 0.45 g curcumin daily and one patient consuming 3.6 g curcumin daily developed diarrhea (National Cancer Institute grades 1 and 2) 1 month and 4 months into treatment, respectively. In the first case, diarrhea was con-trolled with 4 mg of loperamide hydrochloride daily. The other patient withdrew consent from the study before the cause of the diarrhea could be investigated, which resolved after cessation of treatment. One patient consuming 0.9 g curcumin daily experi-enced nausea (National Cancer Institute toxicity grade 2), which resolved spontaneously despite continuation of treatment. Two abnormalities were detected in blood tests, both possibly related to treatment: a rise in serum alkaline phosphatase level was observed in 4 patients, consistent with National Cancer Institute grade 1 toxicity in 2 patients and grade 2 toxicity in 2 patients; serum lactate dehydrogenase rose to 150% of pretreatment values in 3 patients.
Biological Effects of Oral Curcumin. Blood was taken immediately predose or 1 hour postdose on days 1, 2, 8, and 29. Whole blood was incubated for 24 hours in the presence of LPS (10 g/mL). Oral administration of curcumin did not impact on basal PGE2 levels in leukocytes nor did doses of 0.45 to 1.8 g daily alter LPS-induced PGE2 (result not shown). However, consumption of 3.6 g of curcumin daily affected LPS-induced PGE2 levels (Fig. 1). When values obtained immediately pre- or 1 hour postdose on days 1, 2, 8, and 29 were pooled for the 6 patients consuming this dose, PGE2 levels observed postdose (3.2 2.2 ng/mL) were significantly lower (46%, P 0.028; df 59) than those measured immediately predosing (4.5 3.4 ng/mL). As shown in Fig. 1, the difference reached significance on day 1 (62% reduction, P 0.05; df 13) and day 29 of treatment (57% reduction, P 0.01; df 14). Subset analysis revealed no difference between inducible PGE2 levels in sam-ples from the 3 patients in which curcumin was detected com-pared with those in which curcumin was not detected (see below).
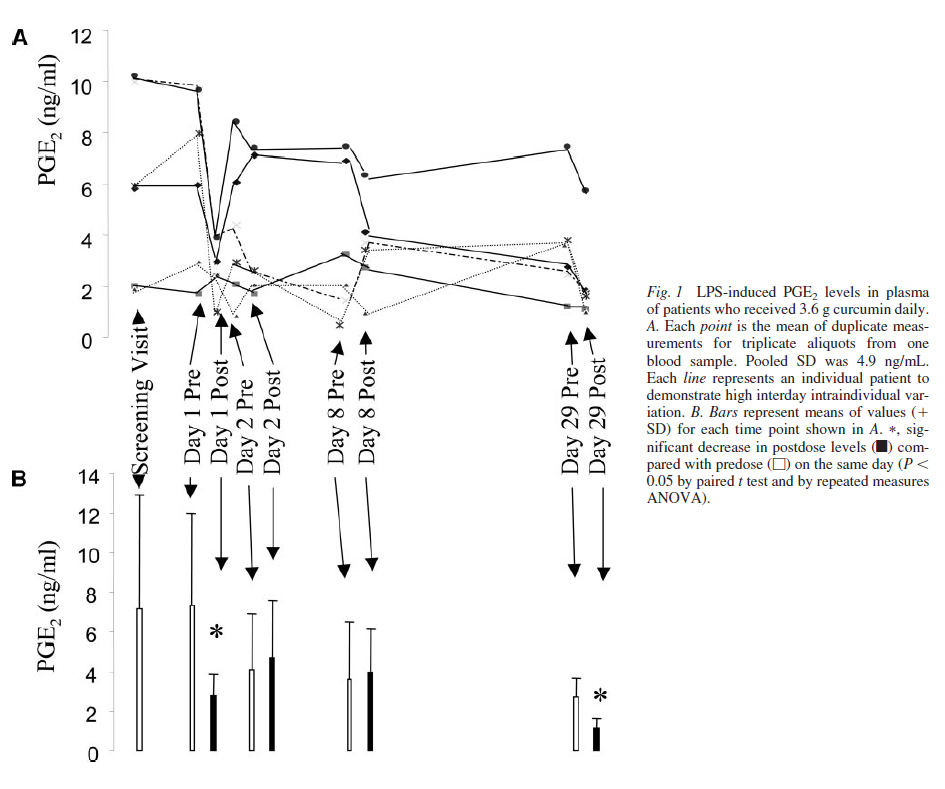
These results suggest that consumption of 3.6 g of curcumin daily is linked with inhibition of PGE2 induction in blood taken postdose compared with blood taken predose. Over-all time-dependent trends were not identified at any dose in basal or LPS-stimulated PGE2.
Total GST activity and M1G levels in leukocytes differed substantially between patients with reasonable reproducibility for each patient across the 4-week study period, as borne out by average coefficients of variation within each patient of 15% and 31% for GST and M1G, respectively (data not shown). Treat-ment-related effects were not observed. Patients were genotyped for GST isoenzymes GSTM1, GSTP1, and GSTT1. Sixty percent of the patients lacked GSTM1. In patients who displayed null genotype for GSTM1, predose levels of leukocytic M1G (pooled for all time points) were 4.4 0.7 per 107 nucleotides, 63% higher than those in patients expressing GSTM1, in whom adduct levels were 2.7 1.1 per 107 nucleotides (P 0.001; df 30).
Levels of Curcumin and Its Metabolites in Blood and Excreta. Curcumin was detected in plasma samples taken 0.5 and 1 hour postdose (see Fig. 2A) from 3 patients consuming 3.6 g of curcumin daily. Semiquantitation afforded the mean of 11.1 0.6 nmol/L for these 3 patients at the 1-hour time point on day 1, and curcumin levels were similar at the 1-hour time points on days 2, 8, and 29 of intervention. The maximum intrasubject variation of plasma curcumin at the 1-hour time point on days 1, 2, 8, and 29 of intervention was 24% of the mean from day 1. Intersubject variation of plasma curcumin at the 1-hour postdosing was between 7% and 41% of the respec-tive means on days 1 and 29 of intervention, respectively. Curcumin was infrequently detected in the plasma of these patients at other time points, and it was not found in plasma from patients who received lower doses of curcumin. The cur-cumin formulation used contained small amounts of desme-thoxycurcumin and bisdesmethoxycurcumin, which explained the two peaks at retention times slightly longer than curcumin shown in Fig. 2A (panel iii). Glucuronides and sulfates of curcumin and desmethoxycurcumin were found in the plasma from all 6 of the patients consuming 3.6 g of curcumin daily at all of the time points studied. The presence in plasma of curcu-min, desmethoxycurcumin, and their glucuronide and sulfate metabolites was confirmed by MS. Semiquantitation of levels in pooled plasma samples yielded 8.9 0.7 and 15.8 0.9 nmol/L for curcumin sulfate and curcumin glucuronide, respec-tively. There were no obvious differences in the levels of these conjugates when plasma samples from the 3 patients in which curcumin was detected were compared with those in which curcumin was not detected.
Analysis of urine suggested the presence of curcumin and the conjugates in all of the samples from patients con-suming 3.6 g of curcumin daily (Fig. 2B). Such chromato-graphic peaks were not seen in any extracts of urine samples from patients on the lower doses. In the 6 patients consuming 3.6 g of curcumin daily, urinary levels varied between 0.1 and 1.3 mol/L (curcumin), 19 and 45 nmol/L (curcumin sulfate), and 210 and 510 nmol/L (curcumin glucuronide; see Fig. 2B and Fig. 3). On the basis of the preclinical data currently available, the presence of curcumin and its metab-olites in the urine was unexpected. Therefore, the assignment…
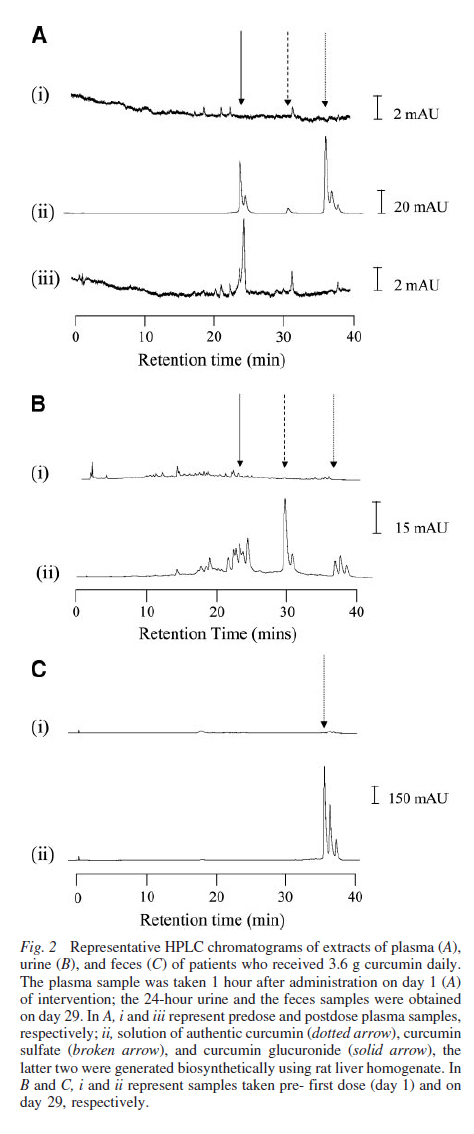
…of HPLC peaks to structures was confirmed by cochromatog-raphy using authentic standards and corroborated by MS with multiple reaction monitoring. These techniques demonstrated the presence of curcumin and curcumin glucuronide, desme-thoxycurcumin…
…and desmethoxycurcumin glucuronide, and curcumin sulfate by compound-specific fragmentation (Fig. 4). Abundant amounts of curcumin were recovered from the feces (Fig. 2C) at all of the dose levels. Curcumin levels in day 8 fecal samples from patients consuming 3.6 g of curcumin daily were between 25 and 116 nmol/g dried feces. Fecal levels of curcumin did not correlate with toxicity in the 2 patients who experienced diarrhea. Trace amounts of cur-cumin sulfate were detected in feces from 3 patients consum-ing 3.6 g of curcumin daily.
Effects of Oral Curcumin on Malignancy. All of the patients enrolled exhibited radiologic evidence of progressive disease before recruitment. No partial responses to treatment were observed. Two patients exhibited stable disease by…
…radiologic criteria after 2 months of treatment, and they remained on treatment for a total of 4 months. The first of these 2 patients (dose level 2) developed progressive disease on her second computed tomography scan. The other patient (dose level 3) demonstrated continued stable disease on com-puted tomography scan after 4 months, but she withdrew consent on account of diarrhea, which she thought was treat-ment-related. Decreases in tumor markers or serum choles-terol were not observed as a result of treatment in any of the patients. Three significant changes in quality of life scores were recorded: 1 patient noticed a significant improvement after 1 month of treatment; and 2 patients deteriorated after 2 months of treatment, both of whom were found to have radiologic progressive disease.
Discussion
The study presented here provides the first report of the systemic parameters of pharmacokinetics and activity of curcu-min that are likely to be of value in Phase II chemoprevention/ anticancer trials of this agent. The results permit the following three conclusions to be drawn regarding oral curcumin in hu-mans: (1) administration of 0.5 to 3.6 g daily for up to 4 months is associated with mild diarrhea as its only discernible toxicity, (2) consumption of 3.6 g of curcumin daily generates detectable levels of parent compound and conjugates in plasma and urine, and (3) consumption of 3.6 g of curcumin daily causes inhibition of PGE2 production in blood leukocytes measured ex vivo. These conclusions lead us to propose that an oral dose of 3.6 g daily is suitable for evaluation in Phase II trials.
The observed safety of curcumin is consistent with previ-ous reports of clinical studies of curcumin and Curcuma extracts (4, 13, 21, 22). Cheng et al. (4) treated patients with premalig-nant conditions with tablets containing pure curcumin for up to 3 months. The authors did not record any treatment-related toxicity at daily doses as high as 8 g, although quality of life was not measured, and blood results were not presented. Levels of curcumin observed at the 1-hour time point after a dose of 3.6 g in the study described here are 1/40 of those described by Cheng et al. (4) when they administered 4 g of curcumin. The reason for the discrepancy between the two studies is unclear; it may be associated with the fact that the formulation used by Cheng et al. (4) consisted of pure chemically synthesized cur-cumin, whereas the formulation used in the trial described here was composed of a purified turmeric extract containing 10% curcuminoids other than curcumin (see Patients and Methods).
Our second conclusion is that consumption of 3.6 g of oral curcumin daily results in levels of drug and conjugates in plasma near the limit of detection of the assays used. Low systemic bioavailability after oral dosing is consistent with findings in preclinical models (9, 12, 23) and in humans (4, 13, 21, 22). In our laboratory, the adenoma-suppressing activity of dietary curcumin (0.2%, equivalent to 300 mg/kg per day) in the APC Min mouse model was associated with a mean level of 111 nmol/g curcumin in the mucosa of the small intestine (23). The curcumin concentration in the plasma of these mice was close to the limit of detection (5 pmol/mL), irrespective of the dietary doses (0.1%, 0.2%, and 0.5%) studied. This result sug-gests that intestinal mucosal levels of curcumin may not be reflected by its systemic levels. Efficient metabolism of curcu-min, especially glucuronidation and sulfation, may explain its poor systemic availability when administered via the oral route (4, 13, 15) and is compatible with the finding of metabolites in the plasma of all of the patients consuming 3.6 g daily, although parent compound was detected in the plasma of only half of the patients consuming this dose. Measurement of compliance is increasingly perceived to be an important component of inter-vention trials (24). The consistent presence of curcumin and its conjugates in urine observed here in patients consuming 3.6 g of curcumin daily is of relevance to the potential clinical advance-ment of curcumin as a chemopreventive agent. Urinary analysis of drug-derived species constitutes an easily accessible and reproducible test for ensuring general compliance.
Of the three potential biomarkers of the systemic activity of curcumin explored in this study, levels of M1G and GST were unaffected by curcumin. These results indicate that leukocyte M1G and GST are not useful indicators of the systemic activity of curcumin in humans due to lack of effect at the low systemic levels of curcumin that result from oral dosing. Interestingly, consistent with a previous observation in patients with advanced colorectal cancer (13), those who lacked GSTM1 expression, a common phenotype in Caucasian populations, had higher leu-kocyte M1G levels than GSTM1-expressing patients. Although GST- is not the most abundant of the GST isoenzyme classes expressed in human lymphocytes (19, 20), this observation suggests that lack of GSTM1 activity may be of direct relevance to the detoxification of endogenous and exogenous oxidants, which cause modification of bases in DNA.
In contrast to M1G and GST, the inducibility of PGE2 pro-duction in whole blood ex vivo may represent a useful tool for assessing the systemic activity of curcumin. COX-2 is an important pharmacological target for nonsteroidal anti-inflammatory drugs, selective COX-2 inhibitors, and polyphenolic agents derived from the diet (25). Because COX-2 is thought to play a pathogenic role in the carcinogenesis of many tissues, its pharmacological modu-lation holds implications for cancer prevention and treatment (11, 25). Previous work in our laboratory on the assay described here suggested that at least part of the effect of curcumin on inducible PGE2 production in human blood can be attributed to inhibition of COX2 transcription (11). This phenomenon was also observed in human-derived colon cells cultured in vitro, and inhibition was demonstrated to be the corollary, at least in part, of the inhibition of the nuclear factor- B–activating enzymes IKK- / by curcumin (10, 15). A mechanism analogous to that described in colon cells in vitro may operate in human leukocytes exposed to curcumin. Remarkably, the effect of curcumin described here was associated with plasma levels detected in the 10 8 mol/L range, less than a hundredth of the concentration of curcumin shown in vitro to elicit an effect in blood or colon cells (10, 11, 15). Frustratingly, exper-iments designed to study the effect of submicromolar concentra-tions of curcumin in cells in vitro were limited by the inhibitory activity of the solvent (dimethyl sulfoxide) on PGE2 production (11). The finding that a difference between predose and postdose was not observed on days 2 and 8 of treatment demonstrates the limitations of this assay as a biomarker of activity on account of the high interday intraindividual variation. Therefore, we suggest that incorporation of this biomarker into Phase II clinical trials of curcumin should use multiple time points and a specified minimum cohort size to account for this variability in the power calculations. Measurement of PGE2 levels may be considered in target tissue as a biomarker that may reflect potential anticancer activity and in blood as an indicator of systemic activity and as a potential “surrogate” for the target tissue.
It should also be noted that curcumin sulfate and products of metabolic reduction of curcumin also inhibited PGE2 production in colon cells in vitro, although their inhibitory potency appeared lower than that of parent curcumin (15). Interestingly, some studies have suggested that curcumin elicits systemic effects relevant to the chemoprevention of cancer in hepatic and mammary tissues of animals, despite attainment of levels of curcumin in these tissues that are in the 10 9 to 10 8 mol/L range (26, 27).
The optimization of Phase I clinical trials of diet-derived putative chemopreventive agents in patients with advanced solid tumors is currently under development in recognition of the fact that the molecular targets of chemoprevention and chemother-apy are often similar or identical (28). Results from pilot studies, such as the clinical trial described here or recent reports on green tea extract (29) or soy isoflavones (30), demonstrate the feasibility of measuring potential biomarkers of pharmacody-namic effects in addition to assessment of toxicity and pharma-cokinetics. Such data improve the planning process for Phase II trials in the prevention or treatment of cancer.
The longstanding general view that the “point of failure” for the clinical development of oral curcumin as an agent directed at the prevention or therapy of cancer in tissues outside the gastrointestinal tract will be its low systemic bioavailability should be reviewed in light of the results presented here. Defi-nite conclusions regarding the optimum dose for targeting the gastrointestinal mucosa cannot be drawn from the study pre-sented here; a separate dose de-escalation trial has been con-ducted recently in patients with resectable cancers based on the assumption that doses 3.6 g daily may be efficacious. The findings of the study presented here lead us to conclude that the systemic pharmacological properties of a daily dose of 3.6 g of curcumin are suitable for its evaluation in the prevention of malignancies at sites other than the gastrointestinal tract.
References
- Kelloff GJ, Crowell JA, Hawk ET, et al. Strategy and planning for chemopreventive drug development: clinical development plans II. J Cell Biochem 1996;63:54 –71.
- Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res 1995;55:259 – 66.
- Kawamori T, Lubet R, Steele VE, et al. Chemopreventative effect of curcumin, a naturally occurring anti-inflammatory agent, during the pro-motion/progression stages of colon cancer. Cancer Res 1999;59:597– 601.
- Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 2001;21:2895–900.
- Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987;73:29 –31.
- Piper JT, Singhal SS, Salameh M, Torman RT, Awasthi YC, Awasthi S. Mechanisms of anticarcinogenic properties of curcumin: the effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int J Biochem Cell Biol 1998;30:445–56.
- Susan M, Rao MNA. Induction of glutathione S-transferase activity by curcumin in mice. Arzneimittel-Forschung (Drug Res) 1992;42:962– 4.
- Jovanovic SV, Steenken S, Boone CW, Simic MG. H-Atom transfer is a preferred antioxidant mechanism of curcumin. J Am Chem Soc 1998;121:9677– 81.
- Sharma RA, Ireson CR, Verschoyle RD, et al. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA ad-ducts in rat liver and colon mucosa: Relationship with drug levels. Clin Cancer Res 2001;7:1452– 8.
- Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF- B activation via the NIK/ IKK signalling complex. Oncogene 1999;18:6013–20.
- Plummer SM, Hill KA, Festing MFW, Steward WP, Gescher AJ, Sharma RA. Clinical development of leukocyte cyclooxygenase 2 ac-tivity as a systemic biomarker for cancer chemopreventive agents. Cancer Epidemiol Biomark Prev 2001;10:1295–9.
- Holder GM, Plummer JL, Ryan AJ. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978;8:761– 8.
- Sharma RA, McLelland HR, Hill KA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colo-rectal cancer. Clin Cancer Res 2001;7:1894 –900.
- Leuratti C, Singh R, Lagneau C, et al. Determination of malondi-aldehyde-induced DNA damage in human tissues using an immunoslot blot assay. Carcinogenesis 1998;19:1919 –24.
- Ireson C, Orr S, Jones DJL, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res 2001;61:1058 – 64.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: A quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249:7130 –9.
- Harries LW, Stubbins MJ, Forman D, Howard GCW, Wolf CR. Identification of genetic polymorphisms at the glutathione S-transferase Pi locus and association with susceptibility to bladder, testicular and prostate cancer. Carcinogenesis 1997;18:641– 4.
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprevention and drug resistance. Crit Rev Biochem Mol Biol 1995;30:445– 600.
- Tew KD. Glutathione-associated enzymes in anticancer drug resist-ance. Cancer Res 1994;54: 4313–20.
- Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with post-opera-tive inflammation. Int J Clin Pharmacol Ther Toxicol 1986;24:651– 4.
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PSSR. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica 1998;64:353– 6.
- Perkins S, Verschoyle RD, Hill KA, et al. Chemopreventive effi-cacy and pharmacokinetics of curcumin in the Min/ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomark Prev 2002; 11:535– 40.
- Brenner DE. Cancer chemoprevention. Crit Rev Oncol Hematol 2000;33:155– 6.
- Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci 2003; 24:96 –102.
- Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol 1998;55:1555– 62.
- Pereira MA, Grubbs CJ, Barnes LH, et al. Effects of the phyto-chemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis 1996;17:1305–11.
- Gescher A, Sharma RA, Steward WP. Cancer chemoprevention by dietary constituents: A salutary tale of failure and promise. Lancet Oncol 2001;2:371–9.
- Pisters KMW, Newman RA, Coldman B, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol 2001;19:1830 – 8.
- Takimoto CH, Glover K, Huang XK, et al. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones admin-istered to individuals with cancer. Cancer Epidemiol. Biomark Prev 2003;12:1213–21.
